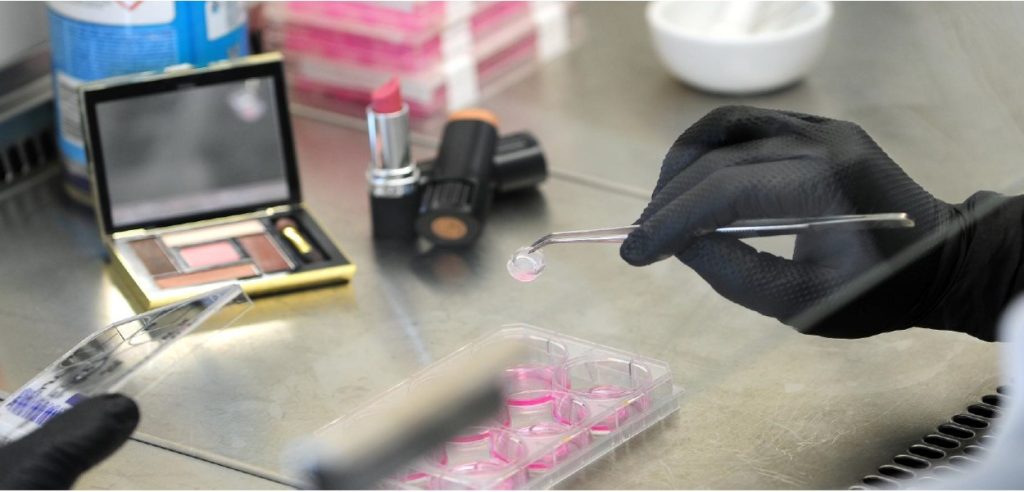
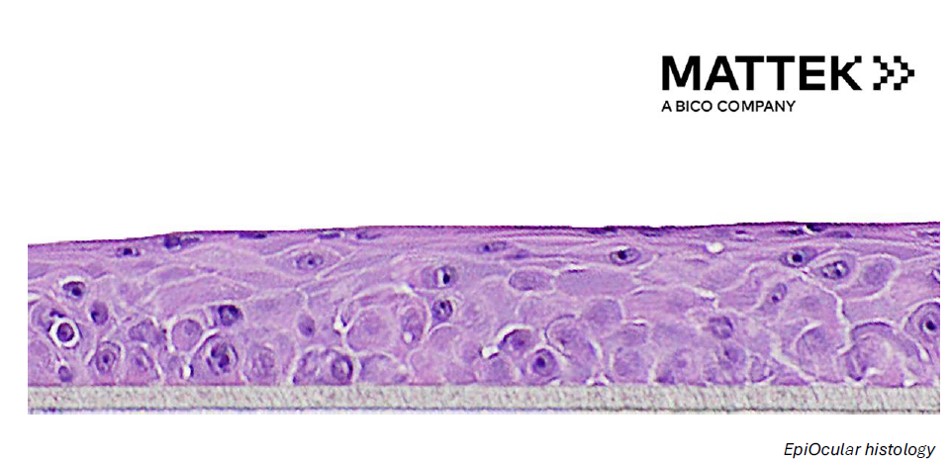
Reconstructed human cornea-like epithelial tissue model (EpiOcular) for the toxicity testing of chemicals, cosmetics, personal care, and pharmaceutical products.
The workshop will provide an overview of the use of in vitro 3D reconstructed tissue model EpiOcular (by MatTek) in toxicology for hazard and risk assessment. The program includes a short presentation on the reconstructed human cornea-like epithelial tissue model, practical hands-on training in the EpiOcular Eye Irritation Test (EIT) according to OECD test guideline 492 (OECD TG 492), and discussion. EpiOcular is a ready-to-use, highly differentiated 3D tissue model consisting of normal, human epidermal keratinocytes (NHEK) which have been cultured in serum-free medium to form a stratified, squamous epithelium, similar to in vivo human corneal epithelium. EpiOcular provides a non-animal model to assess ocular irritation, formulation toxicology, ocular inflammation, and other toxicological endpoints. EpiOcular is most commonly used for ultra-mildness testing and ocular irritation studies. EpiOcular has been validated for the Eye Irritation Test as part of OECD TG 492 and is widely used to meet regulatory testing requirements for testing raw materials and finished products and product optimization studies.
The workshop is suitable for anyone who would like to practice the method, consult the specific problem, and receive valuable information, as well as those who are just considering the use of in vitro models in their research.
- Participation in this workshop is free of charge for registered participants of 3rd NCIM.
- The number of participants is limited to 40 persons.
- All participants receive personalized certificates of attendance.